Challenge: Purification of therapeutics produced in conventional expression systems such as bacterial and mammalian systems, while not without their own issues, have been well established for many years. Newer systems such as the increasingly popular tobacco model bring different challenges, such as the relative toughness of plant cell walls and the abundance of photosynthetic proteins in the cells. Also while some plant proteins can be secreted, this is only to the tissue or spaces within the plant structure itself meaning the whole leaf must be totally distressed. Further to this many plants have antiherbivore defence chemicals. Nicotine is present in many plants at low levels, but in Nicotinia sp. it is present at ~3% of the dry weight of the leaf. Obviously, given its addictive nature it is imperative that as much nicotine as possible is removed. Much of it is removed as a solute eluting off the first Protein A and subsequent columns and buffer exchanges. The normal analytic technique for nicotine analysis would be expected to be Mass Spectrometry. Therefore, as well as proteins, many of which may be impractical to analyse on a MS there is also the removal of a small molecule to analyse and confirm.  Typically, RuBisCO levels might be analysed by gel electrophoresis and nicotine by GC-MS. Both these techniques have some merits, but they add complexity to the analytical process and introduce biases due to their completely different data sets. The former uses labels, usually in the form of Coomassie Blue or silver stains, while the latter needs expensive columns for the analysis and has a very large physical footprint. The two data sets generated are totally disparate and do not lend themselves to an efficient Laboratory Information Management System (LIMS).
Typically, RuBisCO levels might be analysed by gel electrophoresis and nicotine by GC-MS. Both these techniques have some merits, but they add complexity to the analytical process and introduce biases due to their completely different data sets. The former uses labels, usually in the form of Coomassie Blue or silver stains, while the latter needs expensive columns for the analysis and has a very large physical footprint. The two data sets generated are totally disparate and do not lend themselves to an efficient Laboratory Information Management System (LIMS).
Rationale: One of the marginalised areas of the new development of drugs for pandemic response and stratified medicines is analytics. Significant efforts such as the AMP concentrated on the development of new expression systems with only advanced analytical concepts involved in a few cases. In other initiatives for drug manufacture and e.g. synthetic biology, the tendency has been to rely on old analytical technology whilst attempting to development new manufacturing processes. This has logic, as sometimes the demands of a new technique outstrip the capacity to include analytical innovation. This is of course sub-optimal and progress can be held back in the former by reliance on inaccuracy or inefficiencies in the latter. If for example, different classes of analyte in the same expression system require multiple analytical platforms this brings in pressure on time to complete analytics and space within the facility. The removal of both these cellular entities is required in the purification workflow and this requires low bias analytics on a single small footprint platform.
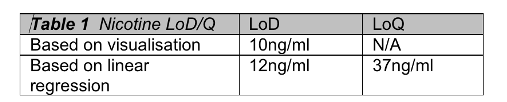
deltaDOT Solution: deltaDOT’s LFII® technology uses no labels, relying on advanced detection and signal processing systems to produce high quality label independent data. Different types of molecules are well known to absorb at different wavelengths and this can be exploited in these analyses. This clear distinction between analytes allows low detection limits to be attained, in particular for chemical analyses. Figure 2 shows the detection of low levels of nicotine in a specialised separation phase. In the higher concentrations there is evidence of enantiomer separation, which can be completely achieved in the appropriate selecting buffer.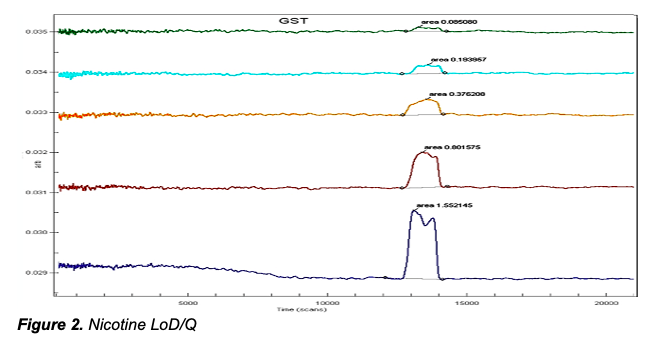
Commercial Applications: The high quality data produced by deltaDOT’s HPCE can be successfully used as an alternative to existing MS techniques in small molecule detection, as follows : – .
• Analyse proteins and small molecules with no labels and on the same system.
• Detect and quantify nicotine in the plant lysate throughout the production workflow.
• Detect enantiomers of nicotine if required.
• Detect and quantify nicotine in column eluents and buffer exchange waste to monitor process efficiency.
• Detect nicotine levels downstream to fill and finish.
• Confirm absence of nicotine in wastewater prior to disposal.
